lateral flow assay development
Lateral flow test strips based on the principles of immunochromatography exist for a wide array of target analytes. Lateral Flow Assay Services.

Ivd Lateral Flow Assay Development
Lateral flow assays LFAs are rapid and inexpensive diagnostic devices that can be used to test for a target substance analyte in a sample.

. Start with sticking the conjugate pad onto the lateral flow backing card. The first infectious disease lateral flow assays were commercialized in the late 1980s identifying the presence of Group A Streptococcus pyogenes collected with throat. The same structure is used whether the product.
Our lateral flow development team is proud of working with a global multi-industry client base. Once it has been done then takes a lateral flow sample pad and sticks it onto the lateral flow backing. Some of the advantages to the LFA.
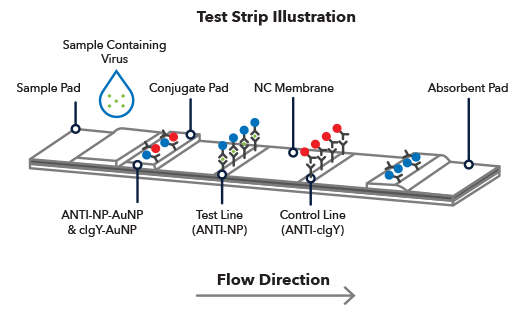
Marginale described in this communi- cation may thus be advantageous. A lateral flow assay LFA is composed of four parts. Lateral flow assays LFAs provide us with a rapid and convenient method of detecting or measuring the concentration of an analyte from a sample.
Conjugate pad on which labeled tags combined with. Core Assay Development Starter Kit includes. The first tests were made for the detection of human chorionic.
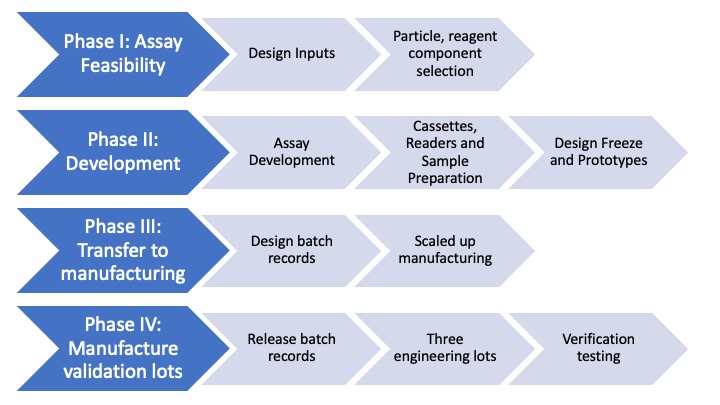
Lateral flow assay development is the successful production of a simple to use diagnostic test for validating the presence or absence of a wide range of pathogens biomarkers and. Lumos assay development follows a standard phased approach with a focus on reducing product risk and matching development effort with the appropriate regulatory requirements. A sample pad which is the area on which sample is dropped.
Lateral Flow Assay Market is expected to reach multi million by 2025 in valuation to 2021 Over the few years the Lateral Flow Assay Market will reach a magnificent spike in CAGR. Standardizing membrane characteristics and optimizing molecular level immunoassay reaction. In this study a highly.
Helping rapid test concepts become a reality. The regional analysis comprehensively done by the researchers highlights key. A prototype of a rapid lateral flow assay for antibody to A.
Bio-Techne is a leading provider of bio-reagents and analytical instrumentation and now provides lateral flow assay development services including. The development of Lateral Flow Immunochromatography Assay can be divided into two levels. 50 Nitrocellulose An.
Soybean mosaic virus SMV is the most common virus in soybean and poses a serious threat to crop production and germplasm recession in many countries worldwide. Lateral flow rapid test development at Abingdon Health typically follows a number of stages with formal reviews at critical points. LFAs are widely used.
Our lateral flow development expertise is paired with our market-leading gold nanoparticle technology or detector label of your choice to deliver an assay to meet your market. The Lateral Flow Assay Components market report is highly structured into a region-wise study. Creative diagnostics lateral flow assay LFA development service will take your assay development from initial idea through to RD and then transfer to trusted partners for bulk.
EXPERIMENTAL Serum Samples Canadian serum and EDTA. The Core Assay Development Starter Kit comes with all that you need to begin optimizing your assay development. By combining experience and passion we.

Nanocomposix Lateral Flow Assay Development Services Podcast
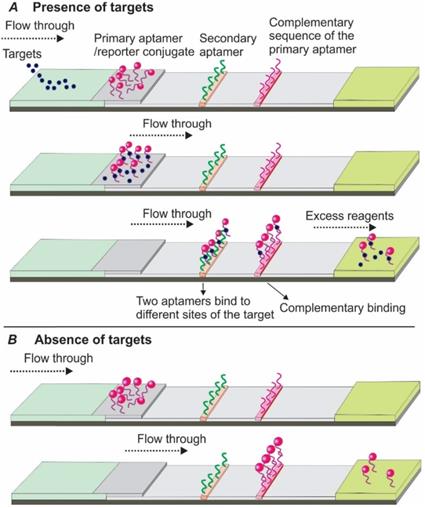
Development Of Nucleic Acid Aptamer Based Lateral Flow Assays A Robust Platform For Cost Effective Point Of Care Diagnosis

Dual Recognition Element Lateral Flow Assay Toward Multiplex Strain Specific Influenza Virus Detection Analytical Chemistry

Ultrasensitive And Highly Specific Lateral Flow Assays For Point Of Care Diagnosis Acs Nano

Lateral Flow Assays Principles Designs And Labels Sciencedirect

Development Of A Smartphone Based Quantum Dot Lateral Flow Immunoassay Strip For Ultrasensitive Detection Of Anti Sars Cov 2 Igg And Neutralizing Antibodies International Journal Of Infectious Diseases

Development Of A Lateral Flow Strip Membrane Assay For Rapid And Sensitive Detection Of The Sars Cov 2 Analytical Chemistry

Recent Advancements In Structural Improvements Of Lateral Flow Assays Towards Point Of Care Testing Sciencedirect

Lateral Flow Assay Development Kit For Lateral Flow Development
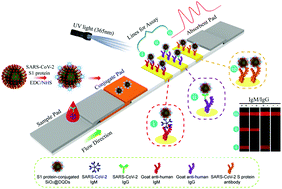
Development Of Spike Protein Based Fluorescence Lateral Flow Assay For The Simultaneous Detection Of Sars Cov 2 Specific Igm And Igg Analyst Rsc Publishing
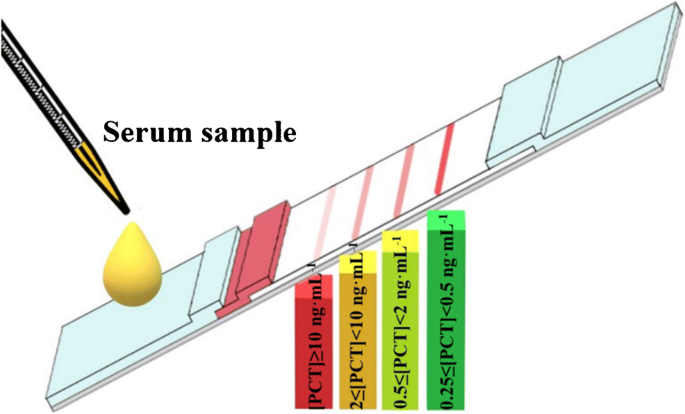
A Semi Quantitative Rapid Multi Range Gradient Lateral Flow Immunoassay For Procalcitonin Springerlink
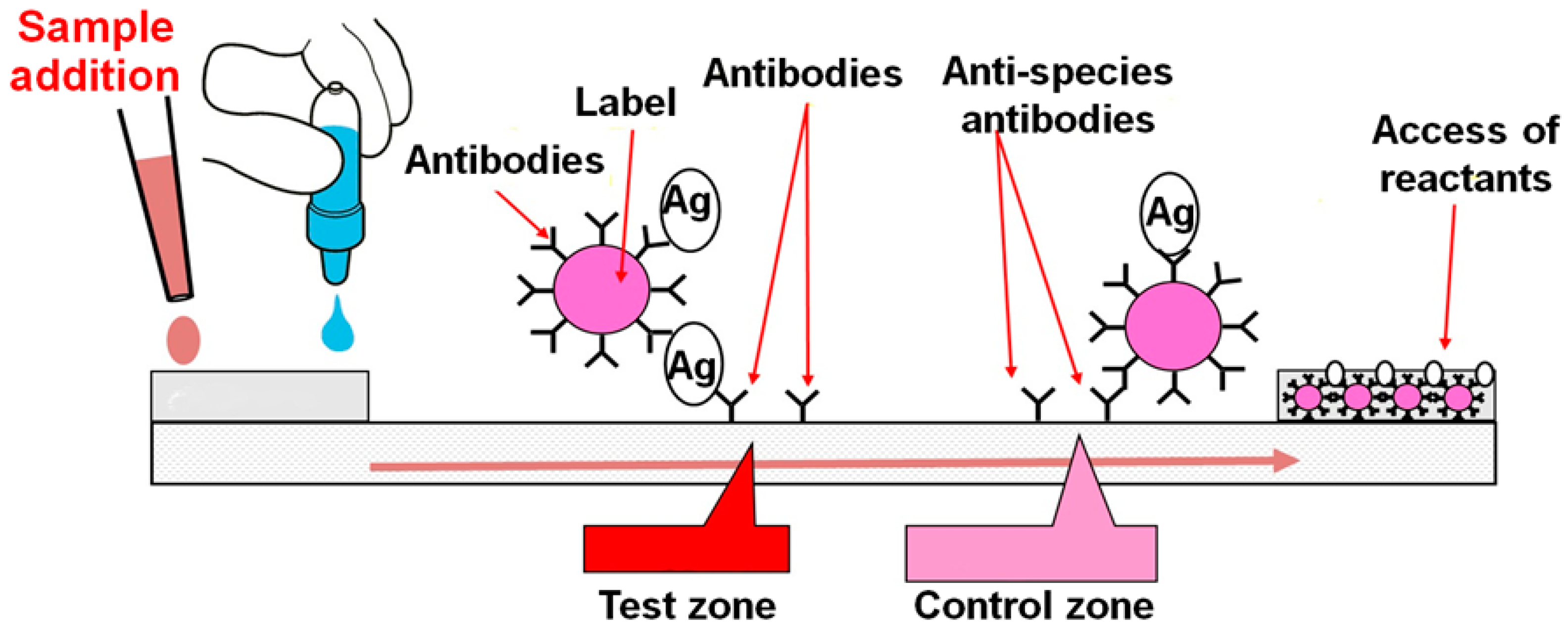
Biosensors Free Full Text Towards Lateral Flow Quantitative Assays Detection Approaches Html
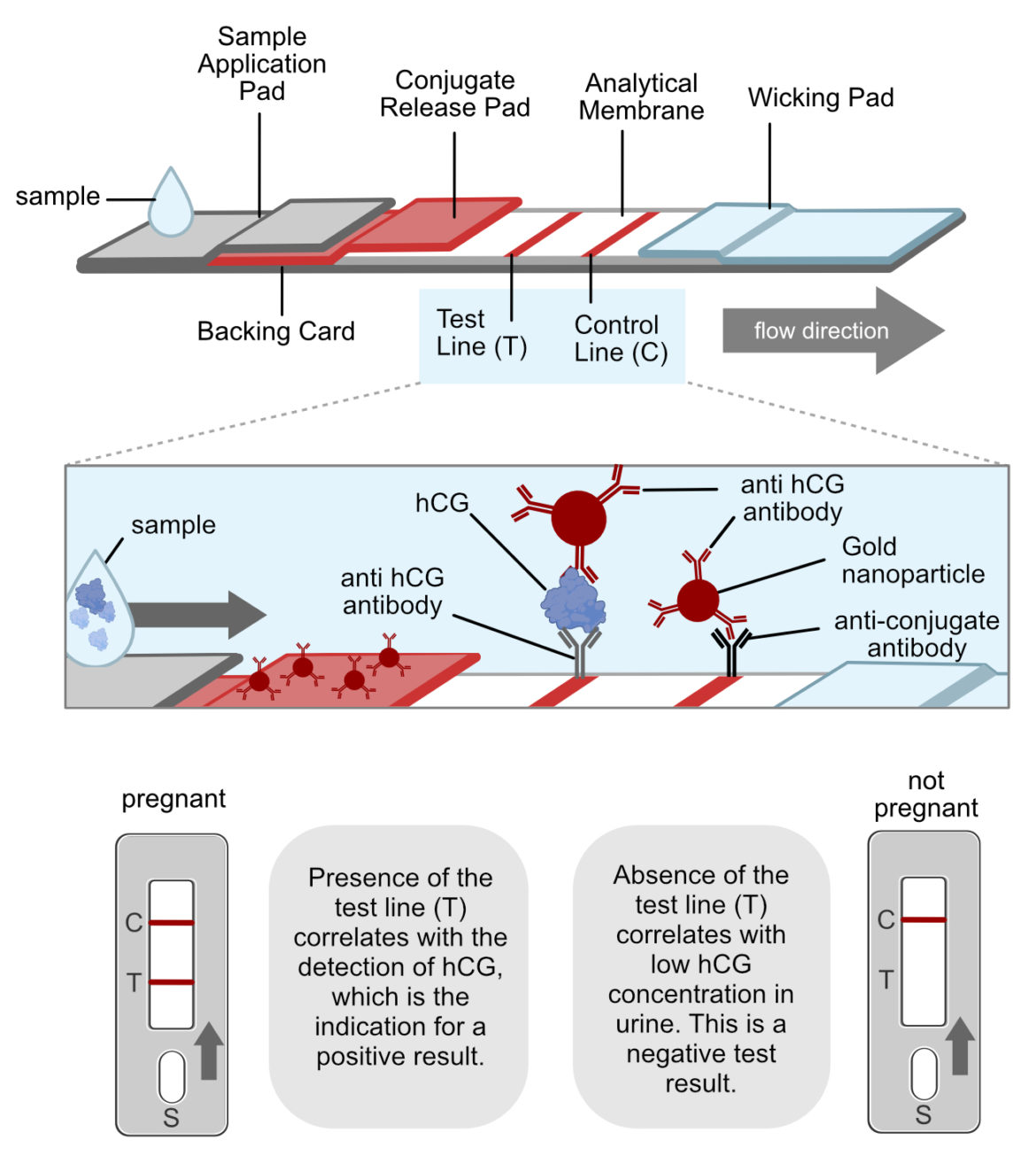
Milenia Hybridetect Lateral Flow Development Platform
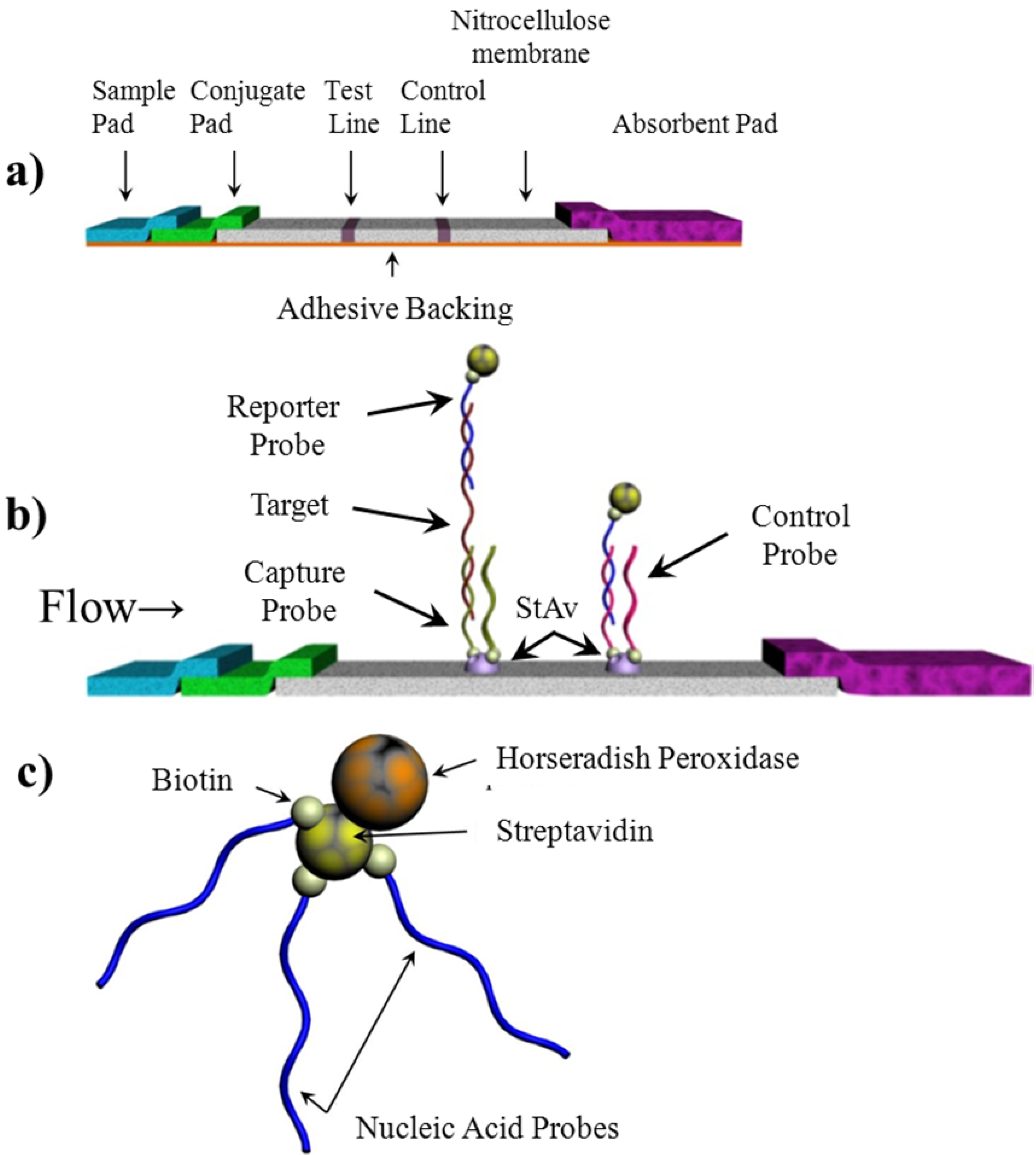
Biosensors Free Full Text Development Of Chemiluminescent Lateral Flow Assay For The Detection Of Nucleic Acids Html
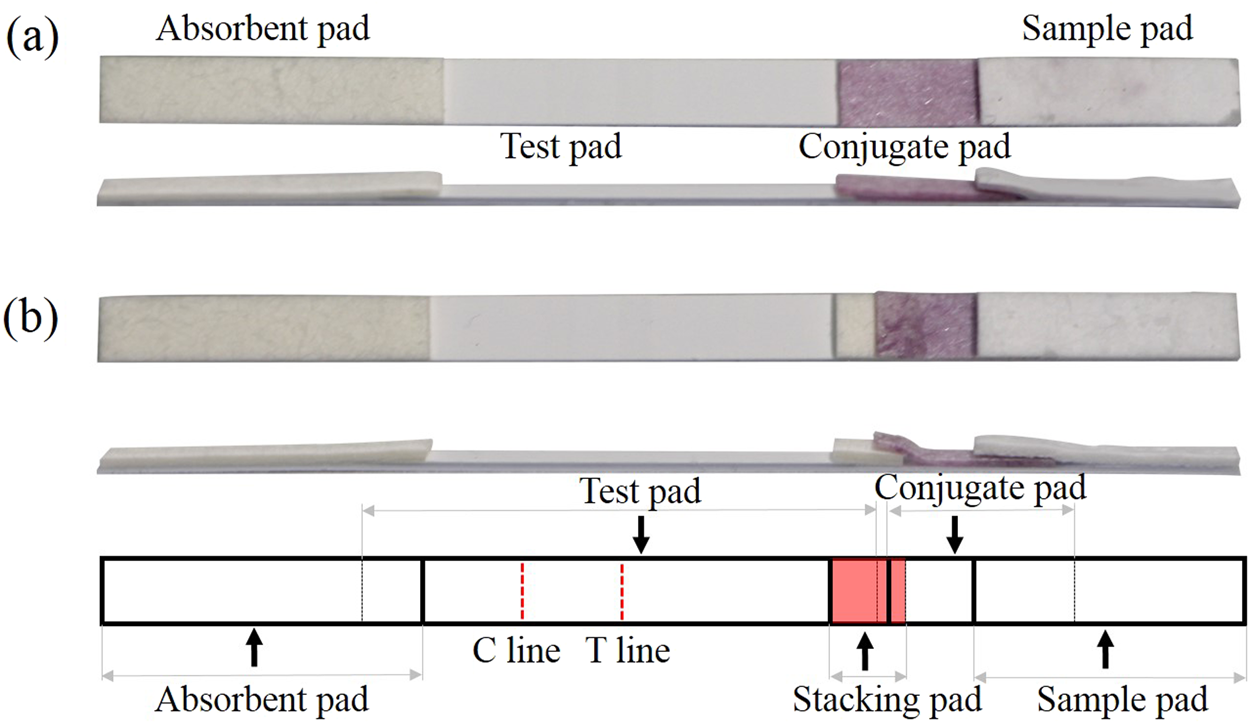
Development A Stacking Pad Design For Enhancing The Sensitivity Of Lateral Flow Immunoassay Scientific Reports
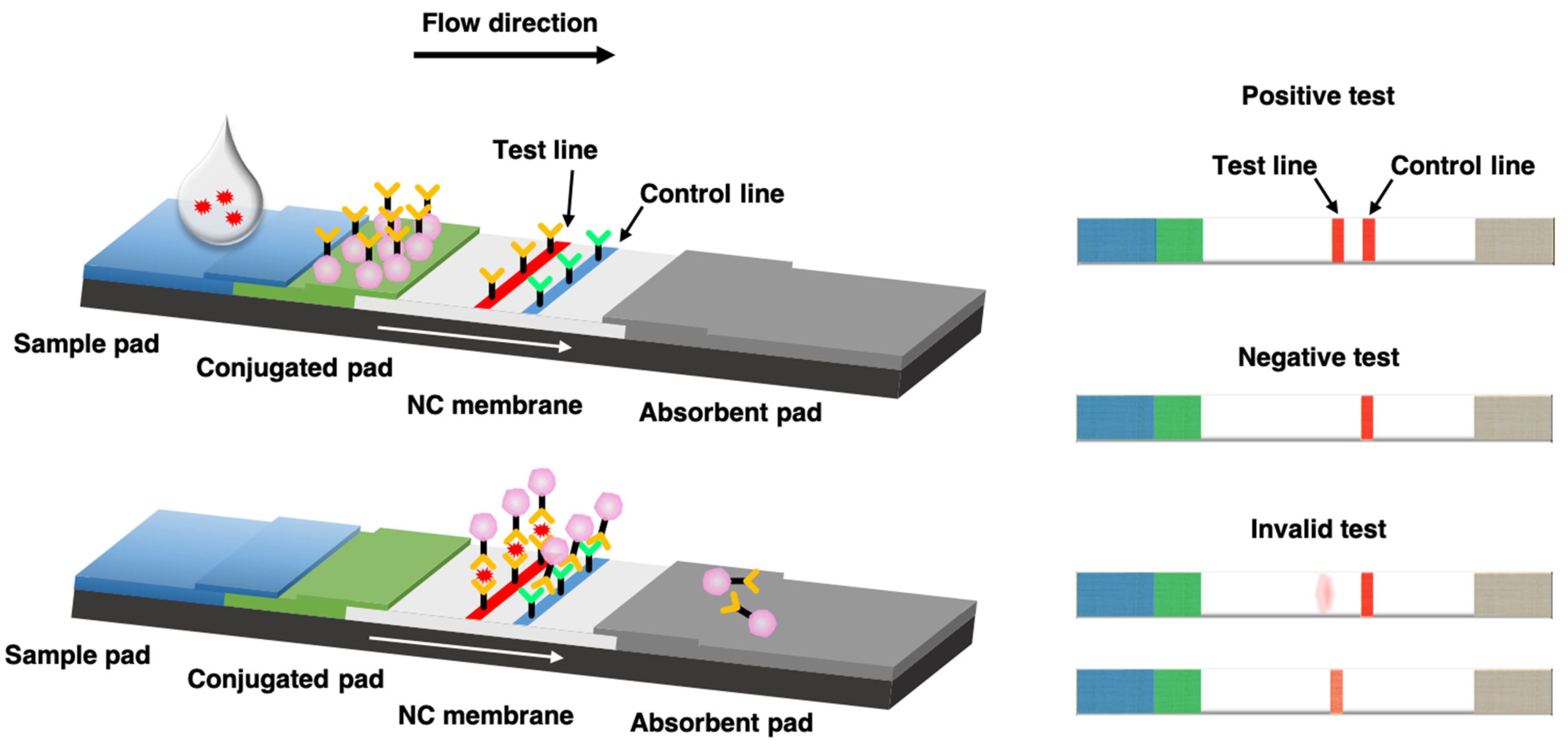
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html